Methylamine hydrochloride: Application, Pharmacokinetics, Toxicity, storage, Preparation
Indication
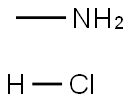
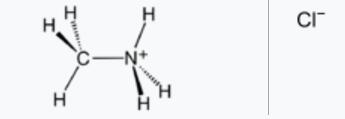
Methylamine hydrochloride[1] is a salt of the organic compound methylamine and hydrochloric acid. It has the chemical formula CH3NH2·HCl and is a white to slightly yellow crystalline powder with a strong odor. Methylamine hydrochloride is used as a reagent in organic synthesis for the preparation of pharmaceuticals, agricultural chemicals, and other industrial products. It can also be used as a precursor to the illegal drug methamphetamine. Methylamine hydrochloride is considered hazardous and requires proper handling and storage.

Figure 1 Appearance of Methylamine hydrochloride
Application
Methylamine hydrochloride has a variety of applications in different industries. It is commonly used as a building block for the synthesis of various organic compounds including pharmaceuticals, pesticides, dyes, and pigments.
In the pharmaceutical industry, it serves as an intermediate for a range of drugs such as anti-inflammatory agents, antihistamines, and local anesthetics. Additionally, it is used in the synthesis of antidepressants, antipsychotics, and other psychoactive drugs. Furthermore, methylamine hydrochloride can synthesize various types of explosives, such as RDX and HMX, due to its explosive properties.
Overall, methylamine hydrochloride plays a crucial role as a starting material or intermediate in many industrial processes, particularly in the pharmaceutical and chemical industries[2-4].
Pharmacokinetics
Methylamine hydrochloride is rapidly absorbed following oral administration. Once absorbed, it likely undergoes metabolism in the liver through the process of N-demethylation to form formaldehyde and ammonia. These metabolites are then further metabolized through various pathways and eventually excreted from the body via the urine.
The half-life of Methylamine hydrochloride is not well established in humans, but it is expected to be relatively short due to its rapid metabolism and elimination. However, this may vary depending on factors such as dose, route of administration, and individual differences in metabolism.
Methylamine hydrochloride is classified as a Schedule I controlled substance in the United States due to its potential for abuse and dependence. Its use should be strictly monitored and regulated by healthcare professionals.
Toxicity
Methylamine hydrochloride is considered a hazardous substance and can be toxic if not handled properly. The toxicity of the compound depends on the concentration, duration of exposure, and route of exposure. Inhalation of methylamine hydrochloride vapors can cause irritation to the respiratory tract, including coughing, wheezing, chest tightness, and shortness of breath. Prolonged or repeated exposure may lead to pulmonary edema, which is characterized by fluid accumulation in the lungs. Skin contact with methylamine hydrochloride can cause irritation, redness, and chemical burns. Eye contact can cause severe irritation, tearing, and corneal damage. Methylamine hydrochloride is also toxic if ingested. Ingestion can cause abdominal pain, vomiting, diarrhea, and in severe cases, circulatory collapse and death. It is important to handle methylamine hydrochloride with care, wear appropriate personal protective equipment, and store the compound in tightly sealed containers in a well-ventilated area away from incompatible substances. In case of accidental exposure, seek medical attention immediately[5].
Storage
Methylamine hydrochloride should be stored in a cool, dry place away from sources of heat and direct sunlight. It is recommended to keep the container tightly closed when not in use to prevent moisture absorption which may result in a decrease in product quality. Additionally, it is important to store Methylamine hydrochloride separately from oxidizing agents and acids to avoid any potential reactions that may occur. Proper storage of this chemical will help ensure its stability and integrity for future use.
Preparation
Methylamine hydrochloride is commonly used in the synthesis of various organic compounds and pharmaceuticals. One common method for synthesizing methylamine hydrochloride involves the reaction of formaldehyde and ammonium chloride.
In this method, formaldehyde is first reacted with ammonium chloride to produce hexamethylenetetramine, which is then treated with hydrochloric acid to form methylamine hydrochloride.
The overall reaction can be represented as:
4NH4Cl + 6CH2O → (CH2)6N4 + 6H2O (CH2)6N4 + 4HCl → 4CH3NH3Cl
Other methods for synthesizing methylamine hydrochloride include the reaction of dimethylamine with hydrogen chloride gas or the reaction of ammonia and methanol in the presence of a catalyst. However, the formaldehyde-ammonium chloride method remains one of the most commonly used methods for industrial-scale production of methylamine hydrochloride.
References
[1] Marvel CS, Jenkins RL. Methylamine hydrochloride[J]. Organic Syntheses, 1923, 3: 67-70.
[2] Radulovic NS, Miltojevic AB, Vukicevic RD. Simple and efficient one-pot solvent-free synthesis of N-methyl imines of aromatic aldehydes[J]. Comptes Rendus Chimie, 2013, 16(3): 257-70.
[3] Simpson LL. Ammonium-chloride and methylamine hydrochloride antagonize clostridial neurotoxins[J]. Journal of Pharmacology And Experimental Therapeutics, 1983, 225(3): 546-52.
[4] Zhu, J., Bieniek, M., & Halsall, T. G. Hydrophobicity of Methylamine and Dimethylamine on the Surface of Aqueous Solutions of Methylamine Hydrochloride and Dimethylamine Hydrochloride Studied by Contact Angle Measurements. The Journal of Physical Chemistry B. 2006, 110(9), 4298-4302.
[5] Sittig, M. (2011). Handbook of toxic and hazardous chemicals and carcinogens. William Andrew.
);You may like
Related articles And Qustion
See also
Lastest Price from Methylamine hydrochloride manufacturers

US $10.00-10.00/ASSAYS2024-05-16
- CAS:
- 593-51-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 tons

US $1.00-0.00/KG2024-05-13
- CAS:
- 593-51-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000KG